

Comparative study of antibacterial activity and stability of D-enantiomeric and L-enantiomeric bovine NK-lysin peptide NK2A. International Journal of Molecular Sciences 2022, 23 Arrow of Time, Entropy, and Protein Folding: Holistic View on Biochirality. Morphology Modulation in Self-Assembly of Chiral 2-Hydroxy-2-Phenylacetic Acids in Polymeric Diluents. Baiq Firyal Salsabila Safitri, Eamor M.Journal of Chemical Theory and Computation 2012, 8 Extension of UNRES Force Field to Treat Polypeptide Chains with d-Amino Acid Residues. The Journal of Organic Chemistry 2013, 78 Oligopeptides with Equal Amounts of l- and d-Amino Acids May Prefer a Helix Screw Sense. Yosuke Demizu, Hiroko Yamashita, Norikazu Yamazaki, Yukiko Sato, Mitsunobu Doi, Masakazu Tanaka, and Masaaki Kurihara.The Journal of Organic Chemistry 2015, 80 Macrocyclization of Peptide Side Chains by the Ugi Reaction: Achieving Peptide Folding and Exocyclic N-Functionalization in One Shot. The Journal of Physical Chemistry B 2015, 119 Origin of Helical Screw Sense Selectivity Induced by Chiral Constrained Cα-Tetrasubstituted α-Amino Acids in Aib-based Peptides. Irene Maffucci, Jonathan Clayden, and Alessandro Contini.The Journal of Organic Chemistry 2019, 84 Mixed Macrocycles Derived from 2,6-Diformylpyridine and Opposite Enantiomers of trans-1,2-Diaminocyclopentane and trans-1,2-Diaminocyclohexane. Rafał Frydrych, Katarzyna Ślepokura, Andrzej Bil, Janusz Gregoliński.Supramolecular Assembly and Small-Molecule Binding by Protein-Engineered Coiled-Coil Fibers. Douglas Renfrew, Richard Bonneau, Jin Kim Montclare. Dustin Britton, Julia Monkovic, Sihan Jia, Chengliang Liu, Farbod Mahmoudinobar, Michael Meleties, P.This article is cited by 26 publications. The library of left- and right-handed 1−3 turn alpha-helical compounds reported herein project their amino acid side chains into very different regions of 3D space, constituting a unique and potentially valuable class of novel scaffolds. This hinge-like switching between structures in response to an external cue suggests possible uses in larger structures to generate smart materials. Adding TFE to their aqueous solutions caused a change to bent helical structures with slightly distorted N-terminal α R or α L-helical turns terminated by a Schellman-like motif adjacent to the C-terminal α L or α R-turn. Heterochiral decapeptides comprising two different cyclic pentapeptides (α Lα R or α Rα L) maintain the respective helical handedness of each monocyclic helical turn component but adopt extended or bent helical structures depending on the solvent environment. Homochiral decapeptides comprising two identical cyclic pentapeptides (α Rα R or α Lα L) are continuous alpha-helical structures that are extremely stable to denaturants, degradative proteases, serum, and additives like TFE, acid, and base.
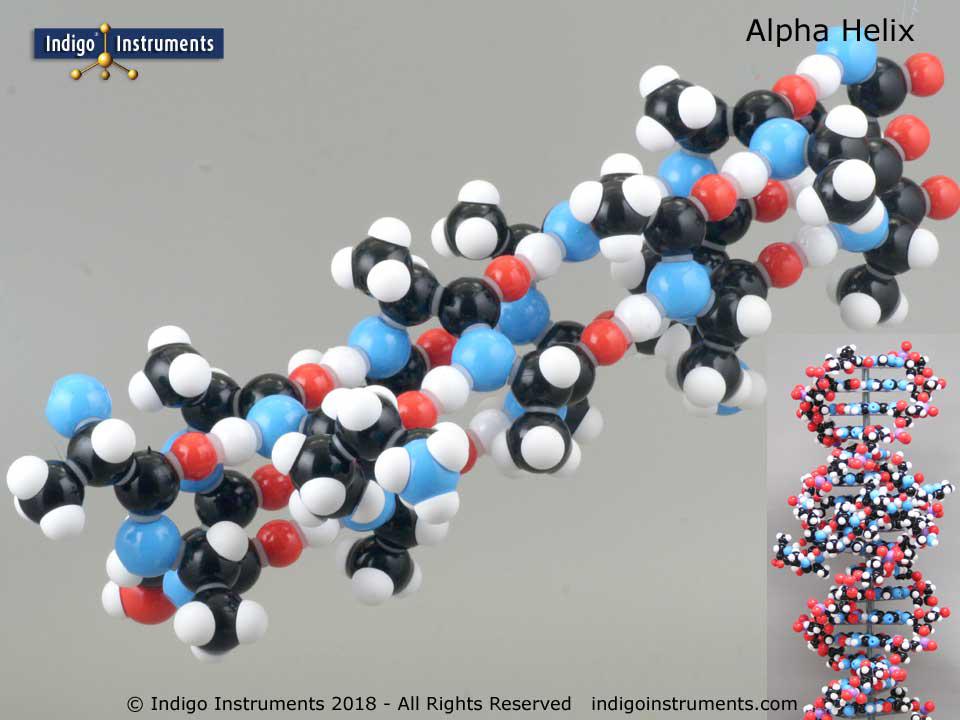
The smallest known water-stable right- (α R) and left- (α L) handed alpha helices are reported, each stabilized in cyclic pentapeptide units containing all L- or all D-amino acids.

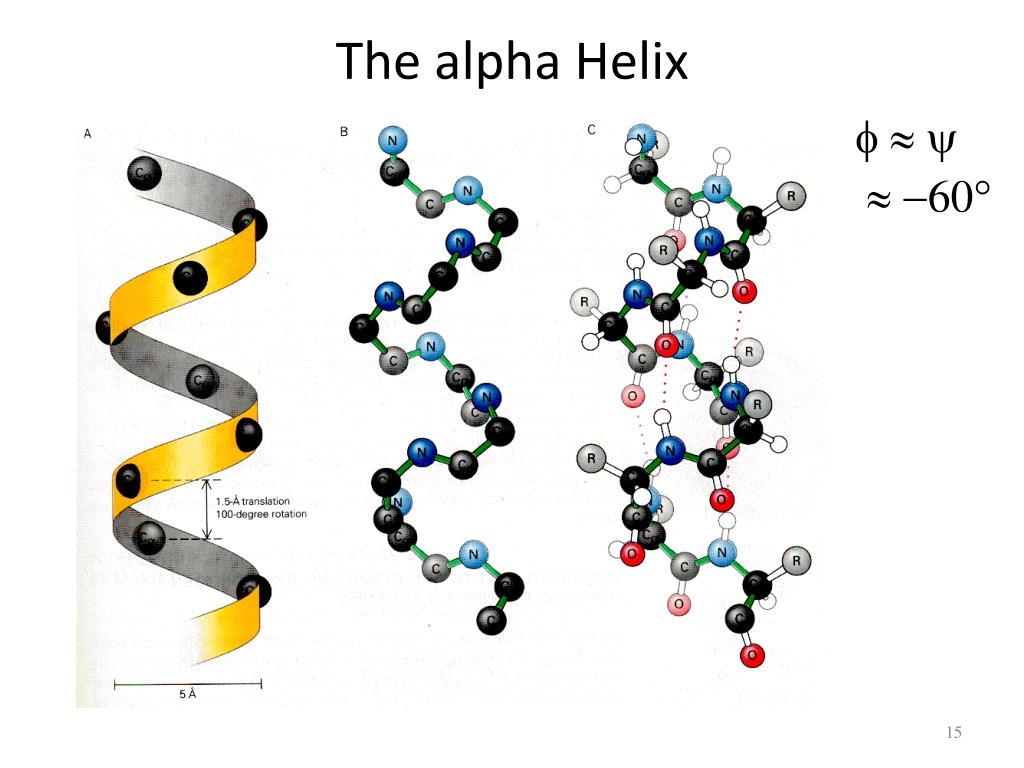
Peptides of 20 amino acids or less corresponding to protein helices do not form thermodynamically stable alpha helices in water away from protein environments. In addition, the effects, or postulated effects, of the alpha-helix dipole on the following phenomena are briefly reviewed: (i) the packing of alpha-helices in proteins (ii) electron, proton and ion conduction along the helix axis (iii) ion transport along multiple helix channels in membranes (iv) flow of electrons guided by helix dipole fields in photosynthetic reaction centres (v) voltage-dependent pore-formation in lipid membranes (vi) packing of helical peptides in water-free crystals (vii) stabilisation and destabilisation of oligopoptides by the interaction between charged side chains and the helix dipole and (viii) a pK shift of a histidine caused by the helix dipole field.Proteins typically consist of right-handed alpha helices, whereas left-handed alpha helices are rare in nature. In about 9 enzymes the active site is situated close to the N-terminus of a helix, in such a manner that the electrical field of the dipole may increase reaction rates. The effects of the alpha-heliz dipole on the distribution of charged residues along the alpha-helix in globular proteins is discussed as well as the helix dipole assisted anion binding by helices in a variety of proteins. The alpha-helix carries a considerable dipole moment, the effect of which can be approximated by placing 0.5-0.7 positive unit charge near the N-terminus and 0.5-0.7 negative unit charge near the C-terminus of the helix.


 0 kommentar(er)
0 kommentar(er)
